
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
Research Background
Fluorescence microscopy has the advantages of less damage to samples, specific labeling, and suitable for in vivo imaging, etc. It has always been the main method in biomedical research.
However, due to the defects of the optical system itself, the unevenness of the optical properties of the biological sample, and the change in the refractive index of the interface between the sample and the microscope infiltration medium, etc., the aberration occurs.
The presence of aberrations makes it difficult for microscopic imaging to achieve diffraction-limited resolution. Aberrations have different effects on different microscopes, but in all cases will lead to a reduction in image contrast and resolution, and as the depth of biological tissue imaging increases, the aberration problem becomes more and more serious, making fluorescence Microscopes cannot image high-resolution deep layers of living organisms.
Adaptive optics (AO) technology corrects dynamic optical wavefront errors by using deformable mirrors (DM) or spatial light modulators (SLM) and other correction elements, thereby improving the imaging performance of the optical system. Therefore, more and more researchers combine AO with fluorescence microscopy to correct dynamic wavefront aberrations and improve imaging qual
A typical AO system includes a wavefront detector, a wavefront controller, and a wavefront corrector, as shown in Figure 1.
Its working principle is:
1) First, the wavefront detector detects the optical wavefront error from the target or the beacon source near the target in real time;
2) The wavefront controller then processes the error and converts it to the control signal of the wavefront corrector and drives it to start working;
3) Finally, the wavefront corrector converts the control signal into a wavefront phase change, compensates and corrects the aberration, restores the wavefront to the state before the distortion, and eliminates the dynamic wavefront error.
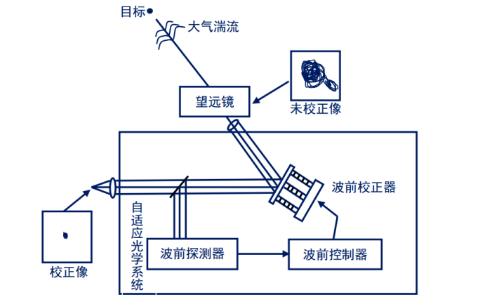
Figure 1 Structure diagram of a typical adaptive optical system
>Wide field imaging microscope
In a wide-field imaging microscope, coherent light or incoherent light is focused on the back focal plane of the objective lens to form approximately parallel illumination light in the sample space. The illumination light illuminates a large range of the sample and excites fluorescence, and finally is detected by the area array Detector imaging.
However, when the area array detector is used for detection, the fluorescence image in the imaging optical path will be directly displayed on the detector, resulting in the image quality being affected by the wavefront aberration.
Therefore, for wide-field imaging microscopes, AO correction of the imaging optical path is necessary.
The wide-field imaging microscope mainly includes ordinary wide-field fluorescence microscope, structured light illumination microscope and single molecule positioning microscope .
>>Ordinary wide-field fluorescence microscope
The illumination method of the ordinary wide-field fluorescence microscope is Kohler illumination, and the aberration of the illumination light path has no effect on the imaging quality, so only the imaging light path needs to be adaptively corrected, as shown in FIG. 2.
Through the model-based optimization algorithm and the phase recovery wavefront sensing mechanism, indirect wavefront detection without wavefront sensing can be achieved, which has a relatively ideal correction effect. The direct wavefront detection method based on the Shock-Hartman wavefront sensor (SHWS) requires the use of guide stars to detect aberrations. Therefore, researchers use artificial guide stars such as fluorescent microspheres, fluorescent proteins, and biological tissues. Backscattered light enables imaging on the order of microns of biological tissue.
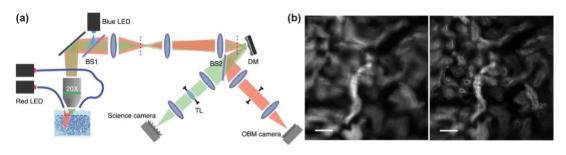
igure 2 Conjugated AO wide-field microscope system and imaging comparison before and after AO correction
>>Structured light microscope
The structured light illumination microscope (SIM) loads the high-frequency information of the object into the detection passband of the optical system through spatial mixing in the frequency domain through structured illumination, thereby achieving super-resolution imaging that breaks through the diffraction limit.
The structured light quality and illumination optical path of SIM are sensitive to aberrations, so the illumination optical path and imaging optical path need to be corrected together, as shown in Figure 3. In 2017, Li et al. used adaptive optical structured light illumination microscope system based on woofer-tweeter double anamorphic mirror and wavefront sensor to achieve faster aberration detection and correction.
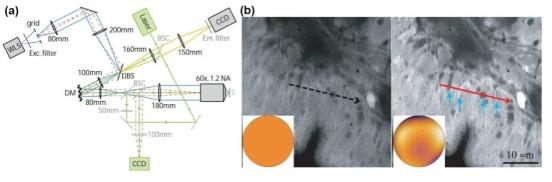
Figure 3 AOSIM system and imaging comparison before and after AO correction
>>Single-molecule localization microscope
Single-molecule localization microscope mainly includes photosensitive localization microscope (PALM) and random optical reconstruction microscope (STORM). By labeling samples with fluorescent molecules with a switching effect, and performing sparse excitation and time-sharing imaging of these fluorescent molecules, single-molecule fluorescence images are obtained, then the centroids of the fluorescent molecules are accurately located, and finally thousands of single-molecules obtained at different times are obtained The molecular positioning information is superimposed to obtain nano-resolution images to achieve super-resolution imaging.
For a single-molecule positioning microscope, only the imaging optical path needs to be adaptively corrected, as shown in 4. In 2013, Burke et al. proposed an optimization algorithm based on image sharpness measurement, which was applied to PALM to achieve aberration correction and imaging quality improvement. Tehrani et al. successively proposed AO-STORM based on genetic algorithm and particle swarm optimization algorithm to realize the real-time correction of the distorted wavefront during the STORM data collection process.
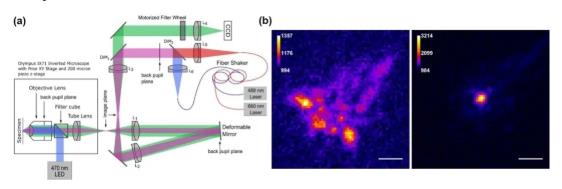
Figure 4 AO single molecule positioning microscope system and imaging comparison before and after AO correction
>Point Scanning Imaging Microscope
In the point scanning imaging microscope, the light emitted by the point light source is expanded and collimated and enters the microscope objective lens in the form of parallel light. The focused light spot focused by the objective lens illuminates a certain point in the sample and excites fluorescence. Fluorescence is collected by the imaging system and then detected by a point detector, and finally imaged by point scanning.
It can be seen that the aberration of the sample will affect the focus point in the illumination light path, dispersing the focus, resulting in the failure to excite the sample or generate unnecessary background light on the defocused surface.
Therefore, for the spot scanning imaging microscope, the correction of the illumination light path is necessary.
Point scanning imaging microscope mainly includes confocal fluorescence microscope, two (multiple) photon fluorescence microscope and stimulated emission loss microscope .
>Confocal fluorescence microscope
The confocal fluorescence microscope uses Laser as the excitation light source. A pinhole is placed in front of the photodetector to conjugate the pinhole to the point light source to filter out stray light. The sample is scanned point by point and layer by layer to achieve high sample height. Resolution three-dimensional imaging.
The dual (multiple) photon fluorescence microscope is based on the nonlinear effect of dual (multiple) photon excitation. The sample is only excited in a small area near the focal point. There is no need to use a pinhole to filter out stray light in front of the detector, so only the illumination light path is needed. Perform aberration correction as shown in Figure 6.
The AO method without wavefront sensor based on aperture segmentation is applied to two-photon fluorescence microscope to realize microscopic imaging of biological tissue with large field of view and large imaging depth. The direct method based on SHWS and DM is applied to the two-photon microscope to realize the direct detection and correction of aberrations in tissues such as Drosophila embryos and mouse brains.
Both the illumination optical path and the imaging optical path of the confocal fluorescence microscope need to be adaptively corrected, as shown in FIG. 5. The Tao team used fluorescent microspheres, fluorescent protein labeling structures, and fluorescent protein centrosomes as guide stars for SHWS to achieve direct measurement of the distorted wavefront and correct it with DM. For indirect wavefront detection, the stochastic parallel gradient descent algorithm is widely used and obtains a good correction effect.
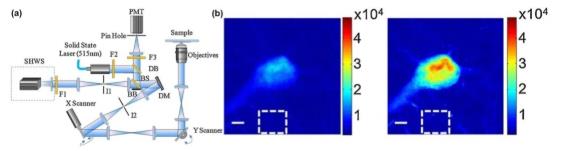
Figure 5 AO confocal microscope system and imaging comparison before and after AO correction
>Double (multiple) photon fluorescence microscope
The dual (multiple) photon fluorescence microscope is based on the nonlinear effect of dual (multiple) photon excitation. The sample is only excited in a small area near the focal point. There is no need to use a pinhole to filter out stray light in front of the detector, so only the illumination light path is needed. Perform aberration correction as shown in Figure 6.
The AO method without wavefront sensor based on aperture segmentation is applied to two-photon fluorescence microscope to realize microscopic imaging of biological tissue with large field of view and large imaging depth. The direct method based on SHWS and DM is applied to the two-photon microscope to realize the direct detection and correction of aberrations in tissues such as Drosophila embryos and mouse brains.
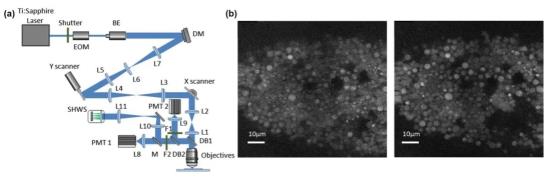
Figure 6 AO two-photon microscope system and imaging comparison before and after AO correction
>Stimulated emission loss (STED) microscope
The stimulated emission loss (STED) microscope suppresses the emission of effective fluorescence by introducing a loss of light in the form of stimulated emission, thereby achieving super-resolution imaging.
STED's three beam paths (excitation light path, depletion light path, and emission light path) are all affected by aberrations, so adaptive correction of all light paths is necessary, as shown in Figure 7.
Gould et al. and Patton et al. used the AO-STED system with two SLMs and DM and SLM correction elements to correct aberrations of different beam paths, and realized thick biological tissues (such as zebrafish retina slices, etc.) ) Three-dimensional super-resolution imaging.
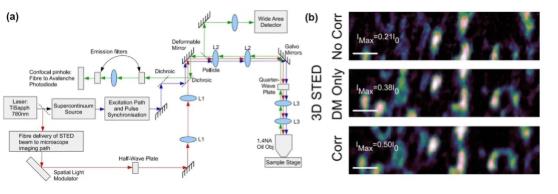
Figure 7 AO-STED microscope system and imaging comparison before and after AO correction
Large field of view, large depth, and fast high-resolution live microscopic imaging provide more accurate image information for biology. In this process, AO technology has played an important role. However, with the continuous development of related technologies, deep dynamic fluorescence imaging of biological tissues has put forward more requirements for the application of AO in fluorescence microscopy.
First of all, the application of AO to the microscopic imaging system will reduce the field of view of high-resolution imaging. To address this problem, the field of microscopic imaging can be expanded by conjugated AO, multi-layer conjugated AO, and a more efficient conjugate correction algorithm. .
Secondly, the wavefront distortion in biological tissue is constantly changing. To detect and correct it in real time, it is necessary to accelerate the detection speed of wavefront distortion and the correction speed of the wavefront corrector to achieve high-speed real-time AO system .
Finally, the study of large field of view and high spatial and temporal resolution imaging of deep tissues is a frontier issue in the current imaging field. In the future, biomedical imaging also puts forward higher requirements for imaging depth. Therefore, it is necessary to further promote deep high resolution fluorescence with the help of AO technology. The development of microscopic imaging.
LET'S GET IN TOUCH

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.